-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer K. Fortin, Jasmine V. Ware, Heiko T. Jansen, Charles C. Schwartz, Charles T. Robbins, Temporal niche switching by grizzly bears but not American black bears in Yellowstone National Park, Journal of Mammalogy, Volume 94, Issue 4, 16 August 2013, Pages 833–844, https://doi.org/10.1644/12-MAMM-A-238.1
Close - Share Icon Share
Abstract
Grizzly bears (Ursus arctos) have been reported as either nocturnal or diurnal in various studies, but have not been known to switch between the 2 times unless disturbed by humans. Black bears (Ursus americanus) are almost solely diurnal in studies unless human influences occur. Because human disturbance is often difficult to control, the relative temporal niche of both species remains ill-defined. Thus, the present study examined bears in Yellowstone National Park (Wyoming) where hunting does not occur, human activities are relatively benign, and bear species are sympatric to determine if niche occupancy was a stable feature of the species. Onset of activity was anticipatory of both sunrise or morning civil twilight (illumination sufficient for human vision) for individuals of either species. The peak hour of activity in black bears was consistently midday, but fluctuated in grizzly bears from midday during early spring, late summer, and fall to evening during late spring and early summer. Black bears did not temporally avoid the times when the more dominant grizzly bears were active. Mean activity levels were higher for male black bears than for both male and female grizzly bears. Together, results suggest that the foraging needs of black bears necessitate ingestion of less-digestible, lower-quality foods requiring longer foraging time during daytime hours, whereas grizzly bears adapt their diet to seasonally available food sources, necessitating greater temporal flexibility.
Overlap in historic geographic ranges of grizzly bears (Ursus arctos) and American black bears (Ursus americanus) occurred over much of North America (Herrero 1972). Thus, temporal niche separation may have been important to the coexistence of these 2 species if it reduced direct competition for resources. Because both species are flexible omnivores, black bears may temporally avoid the more aggressive grizzly bear if daily food availability and harvesting efficiencies are independent of time of day (Mattson et al. 1992, 2005; Gende and Quinn 2004). Alternatively, grizzly bears may be able to use additional resources requiring greater temporal flexibility, such as foraging in nondaylight hours. Indeed, grizzly bears in Yellowstone National Park (Wyoming) have a higher percentage of meat in their assimilated diet (45% ± 22% SD for males and 38% ± 20% for females) than do black bears (23% ± 7%), with grizzly bears feeding more heavily on ungulates and black bears more on insects and small mammals (Fortin et al. 2013). In contrast to the partial dietary separation of meat resources, graminoids and forbs are the primary diet during the summer and whitebark pine nuts are the primary fall diet in good production years for both bear species. Thus, there can be partial or complete competition between the species for several important foods (Fortin et al. 2013). Although black bears may spatially avoid grizzly bears by using more-forested habitat, their habitat use also is highly determined by food availability (Fortin 2011). Switching one's temporal niche would be an efficient mechanism whereby resources are extracted most efficiently.
Temporal niche switching incurs a cost because species are rarely adapted equally to both nocturnal and diurnal conditions (Kronfeld-Schor and Dayan 2003). Thus, niche switching, without external (e.g., human) influence, has been demonstrated in relatively few species (e.g., cotton rats [Sigmodon spp.] and Norway rats [Rattus norvegicus]), because most animals are either completely nocturnal (e.g., owls, many rodents, and most terrestrial amphibians) or completely diurnal (e.g., most birds and most ungulates—Daan 1981; Johnston and Zucker 1983; Green and Bear 1990; Fenn and Macdonald 1995; Roll and Dayan 2002; Ager et al. 2003), but see Hut et al. (2012). More recently, crepuscular species (in the laboratory) such as the fruit fly (Drosophila melanogaster) have been observed to become diurnal in the field, suggesting that niche switching may be more prevalent than previously thought (Vanin et al. 2012). Nevertheless, temporal switching is rarely caused by predation or competition (Kronfeld-Schor and Dayan 2003). Rather, it is more common to observe that animals restrict the amount of time they are active within the same portion of the diel cycle (Schoener 1974). How prevalent niche switching is in bears remains to be determined.
The daily light: dark cycle represents the most predictable geophysical event for synchronizing daily activity patterns (Aronson et al. 1993). This signal has been internalized into the physiology of most organisms via a series of molecular interactions between the so-called “circadian clock genes” (Reppert and Weaver 2002). The body's circadian rhythms are reset each day by light via a process known as entrainment, involving the suprachiasmatic nucleus in the brain. However, clocks have now been identified in many other organs and brain regions (Dibner et al. 2010), allowing additional cues to serve as entrainment signals. For example, food is a robust nonphotic cue that can cause daily patterns of activity to switch from one phase of the daily light: dark cycle to another (Mistlberger 2011). Given the importance to bears of attaining a large body mass prior to hibernation, food entrainment may play an important role in shaping their behavior. In support of this hypothesis, we have recently shown that a switch to nighttime feeding of captive grizzly bears causes a switch to nocturnal activity despite concurrent exposure to natural daylight (Ware et al. 2012).
North American grizzly bears and black bears have been described as typically crepuscular or diurnal (Larivière et al. 1994; Craighead et al. 1995; MacHutchon et al. 1998; Holm et al. 1999; Munro et al. 2006). However, in the Greater Yellowstone Ecosystem, male grizzly bears were nocturnal and crepuscular, whereas female grizzly bears and black bears were crepuscular or diurnal (Holm et al. 1999; Schwartz et al. 2010). Both species switched to nocturnal behavior when external influences (e.g., human activity, predation risks, human hunting, or prey availability) changed (Larivière et al. 1994; MacHutchon et al. 1998; Gibeau et al. 2002; Beckmann and Berger 2003; Nevin and Gilbert 2005; Matthews et al. 2006; Schwartz et al. 2010). Whether individual bears are capable of switching their temporal niche is not clear. Given the apparent flexibility of bears, the present study was undertaken to determine if the extent of temporal switching is similar in both species, and if temporal switching is a product of external influences (e.g., competition) or inherent flexibility (e.g., to maximize niche occupation).
We hypothesized that if activity patterns are controlled primarily by light cues, that activity onsets would be consistently associated with a particular phase of the light: dark cycle (e.g., dawn or dusk); the duration of activity would be longer during fall hyperphagia when bears are driven to maximize food consumption; the peak time of daily activity would be different for black bears if they actively avoided the more aggressive grizzly bears; mean activity levels would be higher for smaller, socially submissive bears (e.g., black bears and female grizzly bears) that often rely on less-digestible and lower-quality foods requiring more foraging effort than the larger and often more carnivorous male grizzly bears; and if an interaction between light- and food-entrainable systems were occurring, then activity patterns would be strongly influenced by the amount and type of food resources (Welch et al. 1997; Rode et al. 2001; Robbins et al. 2004; Fortin et al. 2013).
Materials and Methods
Study area.—We conducted our study from May to October 2007–2009 in the area immediately surrounding Yellowstone Lake, which is part of the Greater Yellowstone Ecosystem. Both bear species used habitats that range from 1,500 m to 3,600 m in elevation (Schwartz et al. 2003). The 10-year (1998–2008) mean high and low temperatures in January were −5.4°C and −17.0°C, respectively, and in July were 23.3°C and 4.6°C, respectively, at Yellowstone Lake (Western Regional Climate Center 2010). Approximately 80% of precipitation typically fell as snow.
Patterns of precipitation and temperature produced predictable vegetation patterns (Marston and Anderson 1991). Yellowstone Lake is usually frozen from December until May or June (Reinhart and Mattson 1990). The west and north drainages of the Yellowstone Lake basin contained small streams draining from low-relief plateaus with lodgepole pine (Pinus contorta) forests and alluvial meadows, whereas higher-relief mountain topography, closed-canopy mixed forests (subalpine fir [Abies lasiocarpa], Engelmann spruce [Picea engelmannii], and whitebark pine [Pinus albicaulis]), and subalpine slopes characterized the east and southeast drainages (Reinhart and Mattson 1990). Alpine tundra occurred at the highest reaches of all major mountain ranges (Patten 1963; Waddington and Wright 1974; Despain 1990). Yellowstone Lake supported native cutthroat trout (Oncorhynchus clarki) and the nonnative lake trout (Salvelinus namaycush).
A diversity of fauna is supported in the Yellowstone Lake area. Large ungulates that populated the area, and were potential prey for bears, consisted of elk (Cervus elaphus), bison (Bison bison), mule deer (Odocoileus hemionus), white-tailed deer (O. virginianus), and moose (Alces americanus). Elk and bison were the most abundant (Knight and Eberhardt 1985). Small mammals included red squirrels (Tamiasciurus hudsonicus), pocket gophers (Thomomys talpoides), Uinta ground squirrels (Urocitellus amatus), and voles (Microtus spp.—Reinhart 1990). Insects included yellow jackets, ants, and cutworm army moths (Euxoa auxiliaris—Reinhart 1990; Robison et al. 2006). Other carnivores included gray wolves (Canis lupus) and coyotes (Canis latrans). Four gray wolf packs overlapped the study area (Smith 2005).
Trapping and handling.—We trapped both grizzly and black bears using culvert traps from fall 2006 through summer 2009. Trapping was performed by members of the Interagency Grizzly Bear Study Team under procedures approved by the Animal Care and Use Committee of the United States Geological Survey, Biological Resources Division, and conformed to the Animal Welfare Act and United States Government principles for the use and care of vertebrate animals used in testing, research, and training. It also conformed to Washington State University's Institutional Animal Care and Use Committee guidelines (permit 3480) and was in accordance with animal care and use guidelines approved by the American Society of Mammalogists (Sikes et al. 2011). Each trapped bear was weighed and sexed, and blood and hair were sampled for stable isotope and mercury analysis and DNA fingerprinting.
We used the telemetry system described and evaluated by Schwartz et al. (2009). We fitted all bears, except dependent young, with Telonics Global Positioning System (GPS) Spread Spectrum collars (Telonics, Inc., Mesa, Arizona) programmed to attempt a GPS fix either every 30 min (1 male black bear, 1 female grizzly bear, and 4 male grizzly bears), 60 min (3 male black bears, 10 female grizzly bears, and 8 male grizzly bears), or 120 min (3 male black bears and 1 female grizzly bear). We excluded collars that malfunctioned due to antenna fatigue (Schwartz et al. 2009) when we calculated fix success. We programmed units to shut off during the expected denning season (15 November–14 April for grizzly bears and 31 October–14 April for black bears). We equipped collars with CR2-A remote drop-off devices (Telonics, Inc.) programmed to release on 1 October of the 1st or 2nd year. In case of CR2-A failure, collars also were equipped with biodegradable cotton canvas spacers. An activity switch with a −15° head to tail sensor also was contained within each transmitter that tallied seconds of switch closure for the 15 min prior to every GPS fix attempt. Activity was recorded as the percentage of total seconds for which the switch was closed during the collection period with a 0.5% resolution. Collars were equipped with a very-high-frequency beacon that operated independently of the GPS device. We flew telemetry flights weekly from late April through mid-October, weather permitting, and downloaded GPS data from the previous week.
Site visits.—After we obtained GPS locations from a telemetry flight, all individuals (both sexes of grizzly bears and black bears) were randomly prioritized for order to sample and a date from the previous week was randomly selected for each bear. We attempted to visit as many locations as possible representing a 24-h period for each collared bear. For bears with fix intervals < 1 h, we visited 1 fix for each hour. For bears with fix intervals ≥ 1 h, we visited every fix. Field surveys occurred within 10 days of the fix to maximize identification of bear sign and to ascertain the type of bear use at each site. All field crews carried handheld GPS units and a very-high-frequency receiver and antenna. If a very-high-frequency signal indicated a collared bear was still present at a GPS location, we delayed our visit until the bear left the area. In 2.8% of cases, we excluded a GPS site to avoid disturbing the bear. We searched a 20-m radius around each GPS location for bear sign. Crews thoroughly searched for scat, hair, bedding, signs of foraging, and carcasses. We identified and recorded all potential foods and whether they showed signs of use by bears.
Activity threshold.—At site visits, bear activity was categorized as active if we saw signs of feeding, and resting if bedding was present. We excluded data points from this analysis that had mixed active and resting bear sign at a single location. Activity counts from transmitters represented the movement of a bear's head up and down during the period just prior to each GPS fix attempt. We used a generalized linear model with consideration for correlation of individual bear activities (PROC GENMOD with GEE—SAS Institute Inc. 2002). Activity was the dependent variable (resting = 0, active = 1) and the predictor variable was the activity count (Schwartz et al. 2009). Each bear was classified as front country or back country based on whether the majority (80%) of their home range did or did not overlap with human developments (as defined by Yellowstone National Park's Bear Management Office) and roads (K. Gunther, Yellowstone National Park, pers. comm.; Spatial Analysis Center 1999).
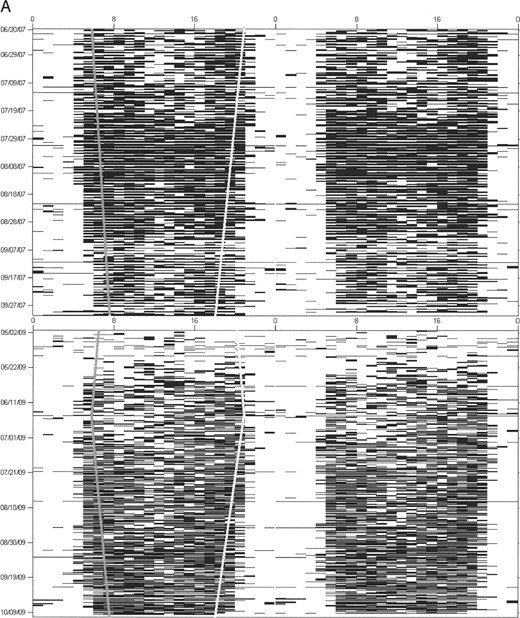
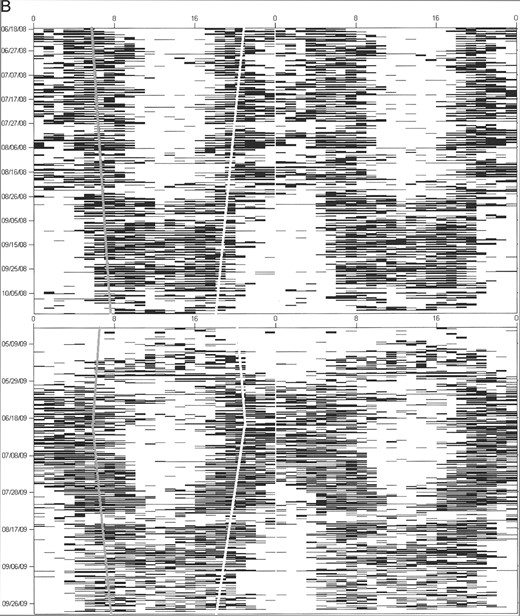
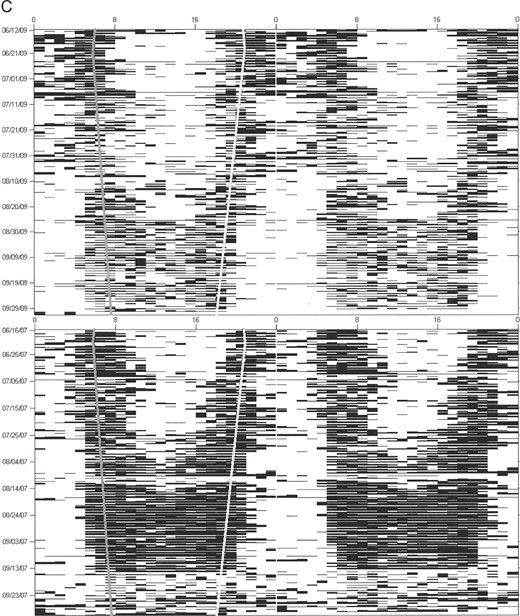
Activity patterns.—We used ClockLab software (Actimetrics, Wilmette, Illinois), run within MATLAB (Mathworks, Natick, Massachusetts), to generate actograms (raster plots of activity data; Fig. 1) for each bear. Actogram-formatted data have been used previously in wild animals to examine temporal activity patterns on a fine scale and to reveal the operation of a circadian clock (Van Oort et al. 2004, 2007). Our own published work has confirmed a high concordance between observer identified (video recorded) and actogram-formatted activity data in captive grizzly bears (Ware et al. 2012). Actograms were double-plotted to better visualize day might transitions and niche switches. Actograms were used for visual inspection of activity patterns to determine the most appropriate classification of time interval. Our sample unit was bear-year, defined as 1 bear's data for 1 active season. Activity patterns for each bear were then analyzed in 2-week intervals from 16 April until 15 November because this was the shortest time interval at which bears switched temporal activity and for which data were available for all bears. However, we recognize that at a 2-week resolution there is the potential for error in identifying when activity changed if it happened at a shorter time interval. Bears were classified as diurnal, crepuscular, or nocturnal during each 2-week time interval based on activity fractions (i.e., the percentage of hours active versus hours of nighttime, twilight, and daytime).
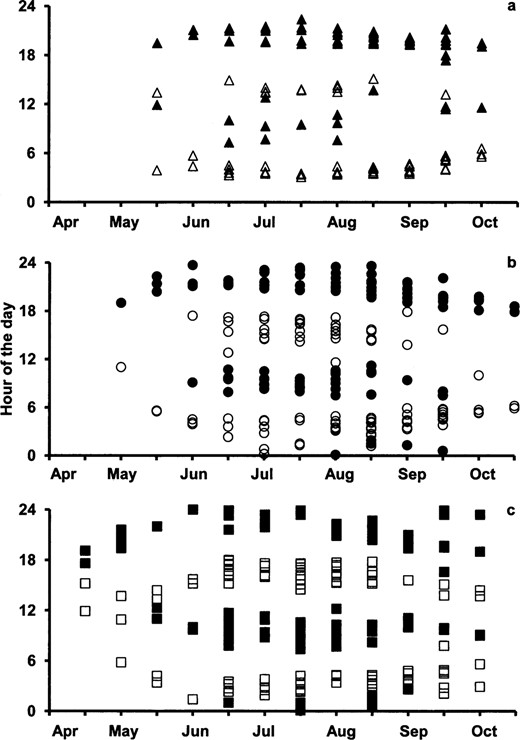
Actogram examples for A) male black bears (Ursus americanus), B) female grizzly bears (Ursus arctos), and C) male grizzly bears in Yellowstone National Park, Wyoming (2007–2009), with activity for each hour plotted on a single line and each line consisting of 2 consecutive days. Although the black bear is clearly diurnal for the entire active season, both female and male grizzly bears shift from nocturnal in the early spring and summer to crepuscular and diurnal in the late summer and fall. Sunrise and sunset are symbolized by gray and white lines, respectively.
The following statistics were obtained for each bear time interval: period (duration of the cycle in hours), phase (time of onset, offset in hours), alpha (activity duration in hours), acrophase (hour of activity rhythm peak based on fitted sine wave), and average daily activity. Period was determined using the Lomb-Scargle periodogram algorithm (MATLAB—Refinetti 2006). The Lomb-Scargle periodogram is optimized for detecting multiple rhythms by repeatedly fitting sine and cosine waveforms to the data that are then normalized and summed to determine the primary frequencies within the data set as described by Van Dongen et al. (1999). Period was determined to both confirm entrainment to a geophysical cue with a 24-h duration and to rule out arrhythmic (random) activity patterns. A period of 24 h or a harmonic thereof (e.g., 12 h) was considered indicative of bona fide entrainment. Phase was calculated by determining points at which the activity profile intersected the activity threshold. Onset was determined as the point when activity rose above the threshold and offset as the point when activity fell below the threshold. The interval between onset and offset (activity duration) was defined as alpha. From the peak of the fitted sine function the acrophase was determined. Mean activity was determined from the raw data. We tested for differences among species, sexes, time intervals, and years using PROC MIXED (SAS Institute Inc. 2002) on diel activity periods (nocturnal, diurnal, or crepuscular); period (duration of the rhythm); phase (time of onset-offset); alpha; acrophase; and mean activity (with activity levels weighted by time interval to account for uneven sample sizes). Astronomical data (sunrise, sunset, and civil twilight) were determined for Old Faithful Lodge, Wyoming (Naval Oceanography Portal 2010).
Feeding time.—Feeding activity was categorized as occurring during daylight or nighttime hours. For feeding activity, data points were excluded when both feeding and daybeds occurred at the same location. Feeding activity was categorized as berry, cambium, earthworms, false-truffles, graminoid, forb, root, whitebark pine nut, ungulate, insect, or other meat. At sites where ungulate carcasses were observed, ArcMAP (Esri, Redlands, California) was used to determine the 1st GPS location at that site, and corresponding activity level, which was assumed to be the time of kill. We tested for differences in the time of feeding for the food categories using chi-square analysis (SAS Institute Inc. 2002).
Results
Site visits.—We deployed 31 collars on 25 individual bears, including 8 female and 11 male grizzly bears and 6 male black bears. Our GPS collars successfully obtained 85.0% of the 105,999 fix attempts. We visited 3,846 locations during our 3-year study and found feeding or resting signs at 1,617 and 1,340, respectively. At 493 of these sites, signs of both feeding and resting were found.
Activity threshold and patterns.—The minimum threshold of activity counts that we used to distinguish between active and resting was 17.3 and was based on bear signs (e.g., beds, digging, or foraging) seen at GPS locations. Activity fractions (e.g., diurnal, crepuscular, or nocturnal) differed between male black bears, female grizzly bears, and male grizzly bears (Table 1). Male grizzly bears used a higher percentage of nighttime hours (48%) than did female grizzly bears (33%), and both were more active at night than were male black bears (19%, F2,184 = 23.64, P < 0.001). The reverse also was true, with male black bears using a higher percentage of available daylight hours (88%) than both male (65%) and female (81%) grizzly bears (F2,184 = 17.83, P < 0.001). The number of twilight hours active did not differ between male (70%) and female (66%) grizzly bears (t = −1.74, P = 0.083), but both were different from that of male black bears (47%; male grizzly bears: t = −6.76, P < 0.001; female grizzly bears: t = −5.48, P < 0.001). We observed no differences in the percentage of nighttime hours active between front-country and back-country male black bears (F1,43 = 1.53, P = 0.222), female grizzly bears (Fl,67 = 2.73, P = 0.103), or male grizzly bears (F1,52 = 0.06, P = 0.814). All black bears were crepuscular or diurnal during all months. Both female and male grizzly bears had some nocturnal activity during late spring and early summer, with nocturnal behavior for males extending further into the fall than for females. Crepuscular activity was evident during the transitional times when grizzly bears shifted between diurnal and nocturnal activity.
Percentage (± SD) of available hours used by male black bears (Ursus americanus) and female and male grizzly bears (Ursus arctos) during daylight, twilight, and nighttime in Yellowstone National Park, Wyoming (2007–2009). Male black bears, female grizzly bears, and male grizzly bears all differed significantly from each other in the percentage of both nighttime and daytime hours used. Both sexes of grizzly bears differed significantly from black bears in the percentage of twilight hours used but did not differ significantly from each other.
| Interval . | Male black bears . | Female grizzly bears . | Male grizzly bears . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | |
| 16–30 April | 2 | 37 ± 37 | 0 ± 0 | 0 ± 0 | ||||||||
| 1–15 May | 1 | 55 | 0 | 0 | 3 | 68 ± 28 | 17 ± 29 | 2 ± 3 | ||||
| 16–31 May | 1 | 81 | 50 | 17 | 3 | 99 ± 2 | 54 ± 25 | 4 ± 6 | 2 | 86 ± 11 | 99 ± 0 | 25 ± 7 |
| 1–15 June | 2 | 98 ± 35 | 25 ± 35 | 4 ± 6 | 5 | 89 ± 24 | 76 ± 24 | 35 ± 39 | 2 | 64 ± 1 | 100 ± 0 | 90 ± 14 |
| 16–30 June | 4 | 86 ± 13 | 4 ± 5 | 17 ± 7 | 6 | 76 ± 23 | 86 ± 22 | 61 ± 44 | 7 | 57 ± 7 | 97 ± 7 | 73 ± 31 |
| 1–15 July | 6 | 83 ± 20 | 57 ± 12 | 20 ± 5 | 8 | 68 ± 22 | 86 ± 22 | 56 ± 37 | 8 | 61 ± 19 | 100 ± 0 | 62 ± 36 |
| 16–31 July | 5 | 85 ± 21 | 69 ± 24 | 28 ± 7 | 10 | 66 ± 24 | 92 ± 17 | 69 ± 39 | 9 | 51 ± 13 | 100 ± 0 | 71 ± 19 |
| 1–15 August | 9 | 85 ± 19 | 60 ± 17 | 25 ± 3 | 12 | 76 ± 25 | 93 ± 15 | 56 ± 36 | 9 | 54 ± 23 | 97 ± 8 | 68 ± 39 |
| 16–31 August | 9 | 96 ± 5 | 63 ± 21 | 24 ± 3 | 11 | 86 ± 21 | 87 ± 20 | 43 ± 30 | 7 | 61 ± 30 | 88 ± 21 | 50 ± 27 |
| 1–15 September | 8 | 99 ± 2 | 69 ± 21 | 23 ± 4 | 10 | 87 ± 29 | 75 ± 35 | 26 ± 23 | 7 | 83 ± 27 | 66 ± 24 | 34 ± 28 |
| 16–30 September | 7 | 92 ± 10 | 72 ± 25 | 18 ± 10 | 8 | 79 ± 37 | 77 ± 24 | 24 ± 21 | 6 | 83 ± 20 | 74 ± 38 | 34 ± 32 |
| 1–15 October | 3 | 79 ± 37 | 1 ± 0 | 9 ± 4 | 4 | 93 ± 14 | 1 ± 0 | 11 ± 8 | 3 | 71 ± 26 | 1 ± 0 | 61 ± 44 |
| 16–31 October | 2 | 98 ± 3 | 60 ± 14 | 10 ± 2 | ||||||||
| X̄ | 88 ± 7 | 47 ± 27 | 19 ± 7 | 81 ± 13 | 66 ± 33 | 33 ± 24 | 65 ± 14 | 70 ± 40 | 48 ± 29 | |||
| Interval . | Male black bears . | Female grizzly bears . | Male grizzly bears . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | |
| 16–30 April | 2 | 37 ± 37 | 0 ± 0 | 0 ± 0 | ||||||||
| 1–15 May | 1 | 55 | 0 | 0 | 3 | 68 ± 28 | 17 ± 29 | 2 ± 3 | ||||
| 16–31 May | 1 | 81 | 50 | 17 | 3 | 99 ± 2 | 54 ± 25 | 4 ± 6 | 2 | 86 ± 11 | 99 ± 0 | 25 ± 7 |
| 1–15 June | 2 | 98 ± 35 | 25 ± 35 | 4 ± 6 | 5 | 89 ± 24 | 76 ± 24 | 35 ± 39 | 2 | 64 ± 1 | 100 ± 0 | 90 ± 14 |
| 16–30 June | 4 | 86 ± 13 | 4 ± 5 | 17 ± 7 | 6 | 76 ± 23 | 86 ± 22 | 61 ± 44 | 7 | 57 ± 7 | 97 ± 7 | 73 ± 31 |
| 1–15 July | 6 | 83 ± 20 | 57 ± 12 | 20 ± 5 | 8 | 68 ± 22 | 86 ± 22 | 56 ± 37 | 8 | 61 ± 19 | 100 ± 0 | 62 ± 36 |
| 16–31 July | 5 | 85 ± 21 | 69 ± 24 | 28 ± 7 | 10 | 66 ± 24 | 92 ± 17 | 69 ± 39 | 9 | 51 ± 13 | 100 ± 0 | 71 ± 19 |
| 1–15 August | 9 | 85 ± 19 | 60 ± 17 | 25 ± 3 | 12 | 76 ± 25 | 93 ± 15 | 56 ± 36 | 9 | 54 ± 23 | 97 ± 8 | 68 ± 39 |
| 16–31 August | 9 | 96 ± 5 | 63 ± 21 | 24 ± 3 | 11 | 86 ± 21 | 87 ± 20 | 43 ± 30 | 7 | 61 ± 30 | 88 ± 21 | 50 ± 27 |
| 1–15 September | 8 | 99 ± 2 | 69 ± 21 | 23 ± 4 | 10 | 87 ± 29 | 75 ± 35 | 26 ± 23 | 7 | 83 ± 27 | 66 ± 24 | 34 ± 28 |
| 16–30 September | 7 | 92 ± 10 | 72 ± 25 | 18 ± 10 | 8 | 79 ± 37 | 77 ± 24 | 24 ± 21 | 6 | 83 ± 20 | 74 ± 38 | 34 ± 32 |
| 1–15 October | 3 | 79 ± 37 | 1 ± 0 | 9 ± 4 | 4 | 93 ± 14 | 1 ± 0 | 11 ± 8 | 3 | 71 ± 26 | 1 ± 0 | 61 ± 44 |
| 16–31 October | 2 | 98 ± 3 | 60 ± 14 | 10 ± 2 | ||||||||
| X̄ | 88 ± 7 | 47 ± 27 | 19 ± 7 | 81 ± 13 | 66 ± 33 | 33 ± 24 | 65 ± 14 | 70 ± 40 | 48 ± 29 | |||
Percentage (± SD) of available hours used by male black bears (Ursus americanus) and female and male grizzly bears (Ursus arctos) during daylight, twilight, and nighttime in Yellowstone National Park, Wyoming (2007–2009). Male black bears, female grizzly bears, and male grizzly bears all differed significantly from each other in the percentage of both nighttime and daytime hours used. Both sexes of grizzly bears differed significantly from black bears in the percentage of twilight hours used but did not differ significantly from each other.
| Interval . | Male black bears . | Female grizzly bears . | Male grizzly bears . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | |
| 16–30 April | 2 | 37 ± 37 | 0 ± 0 | 0 ± 0 | ||||||||
| 1–15 May | 1 | 55 | 0 | 0 | 3 | 68 ± 28 | 17 ± 29 | 2 ± 3 | ||||
| 16–31 May | 1 | 81 | 50 | 17 | 3 | 99 ± 2 | 54 ± 25 | 4 ± 6 | 2 | 86 ± 11 | 99 ± 0 | 25 ± 7 |
| 1–15 June | 2 | 98 ± 35 | 25 ± 35 | 4 ± 6 | 5 | 89 ± 24 | 76 ± 24 | 35 ± 39 | 2 | 64 ± 1 | 100 ± 0 | 90 ± 14 |
| 16–30 June | 4 | 86 ± 13 | 4 ± 5 | 17 ± 7 | 6 | 76 ± 23 | 86 ± 22 | 61 ± 44 | 7 | 57 ± 7 | 97 ± 7 | 73 ± 31 |
| 1–15 July | 6 | 83 ± 20 | 57 ± 12 | 20 ± 5 | 8 | 68 ± 22 | 86 ± 22 | 56 ± 37 | 8 | 61 ± 19 | 100 ± 0 | 62 ± 36 |
| 16–31 July | 5 | 85 ± 21 | 69 ± 24 | 28 ± 7 | 10 | 66 ± 24 | 92 ± 17 | 69 ± 39 | 9 | 51 ± 13 | 100 ± 0 | 71 ± 19 |
| 1–15 August | 9 | 85 ± 19 | 60 ± 17 | 25 ± 3 | 12 | 76 ± 25 | 93 ± 15 | 56 ± 36 | 9 | 54 ± 23 | 97 ± 8 | 68 ± 39 |
| 16–31 August | 9 | 96 ± 5 | 63 ± 21 | 24 ± 3 | 11 | 86 ± 21 | 87 ± 20 | 43 ± 30 | 7 | 61 ± 30 | 88 ± 21 | 50 ± 27 |
| 1–15 September | 8 | 99 ± 2 | 69 ± 21 | 23 ± 4 | 10 | 87 ± 29 | 75 ± 35 | 26 ± 23 | 7 | 83 ± 27 | 66 ± 24 | 34 ± 28 |
| 16–30 September | 7 | 92 ± 10 | 72 ± 25 | 18 ± 10 | 8 | 79 ± 37 | 77 ± 24 | 24 ± 21 | 6 | 83 ± 20 | 74 ± 38 | 34 ± 32 |
| 1–15 October | 3 | 79 ± 37 | 1 ± 0 | 9 ± 4 | 4 | 93 ± 14 | 1 ± 0 | 11 ± 8 | 3 | 71 ± 26 | 1 ± 0 | 61 ± 44 |
| 16–31 October | 2 | 98 ± 3 | 60 ± 14 | 10 ± 2 | ||||||||
| X̄ | 88 ± 7 | 47 ± 27 | 19 ± 7 | 81 ± 13 | 66 ± 33 | 33 ± 24 | 65 ± 14 | 70 ± 40 | 48 ± 29 | |||
| Interval . | Male black bears . | Female grizzly bears . | Male grizzly bears . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | n . | Daylight . | Twilight . | Nighttime . | |
| 16–30 April | 2 | 37 ± 37 | 0 ± 0 | 0 ± 0 | ||||||||
| 1–15 May | 1 | 55 | 0 | 0 | 3 | 68 ± 28 | 17 ± 29 | 2 ± 3 | ||||
| 16–31 May | 1 | 81 | 50 | 17 | 3 | 99 ± 2 | 54 ± 25 | 4 ± 6 | 2 | 86 ± 11 | 99 ± 0 | 25 ± 7 |
| 1–15 June | 2 | 98 ± 35 | 25 ± 35 | 4 ± 6 | 5 | 89 ± 24 | 76 ± 24 | 35 ± 39 | 2 | 64 ± 1 | 100 ± 0 | 90 ± 14 |
| 16–30 June | 4 | 86 ± 13 | 4 ± 5 | 17 ± 7 | 6 | 76 ± 23 | 86 ± 22 | 61 ± 44 | 7 | 57 ± 7 | 97 ± 7 | 73 ± 31 |
| 1–15 July | 6 | 83 ± 20 | 57 ± 12 | 20 ± 5 | 8 | 68 ± 22 | 86 ± 22 | 56 ± 37 | 8 | 61 ± 19 | 100 ± 0 | 62 ± 36 |
| 16–31 July | 5 | 85 ± 21 | 69 ± 24 | 28 ± 7 | 10 | 66 ± 24 | 92 ± 17 | 69 ± 39 | 9 | 51 ± 13 | 100 ± 0 | 71 ± 19 |
| 1–15 August | 9 | 85 ± 19 | 60 ± 17 | 25 ± 3 | 12 | 76 ± 25 | 93 ± 15 | 56 ± 36 | 9 | 54 ± 23 | 97 ± 8 | 68 ± 39 |
| 16–31 August | 9 | 96 ± 5 | 63 ± 21 | 24 ± 3 | 11 | 86 ± 21 | 87 ± 20 | 43 ± 30 | 7 | 61 ± 30 | 88 ± 21 | 50 ± 27 |
| 1–15 September | 8 | 99 ± 2 | 69 ± 21 | 23 ± 4 | 10 | 87 ± 29 | 75 ± 35 | 26 ± 23 | 7 | 83 ± 27 | 66 ± 24 | 34 ± 28 |
| 16–30 September | 7 | 92 ± 10 | 72 ± 25 | 18 ± 10 | 8 | 79 ± 37 | 77 ± 24 | 24 ± 21 | 6 | 83 ± 20 | 74 ± 38 | 34 ± 32 |
| 1–15 October | 3 | 79 ± 37 | 1 ± 0 | 9 ± 4 | 4 | 93 ± 14 | 1 ± 0 | 11 ± 8 | 3 | 71 ± 26 | 1 ± 0 | 61 ± 44 |
| 16–31 October | 2 | 98 ± 3 | 60 ± 14 | 10 ± 2 | ||||||||
| X̄ | 88 ± 7 | 47 ± 27 | 19 ± 7 | 81 ± 13 | 66 ± 33 | 33 ± 24 | 65 ± 14 | 70 ± 40 | 48 ± 29 | |||
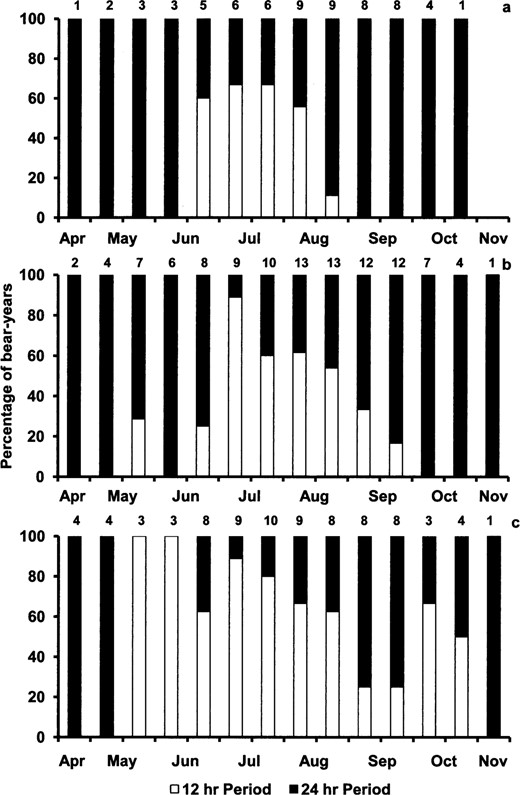
Nocturnal and diurnal activity corresponded with 24-h periods, whereas crepuscular activity was reflected in 12-h periodicities (Fig. 2). We observed a difference in period, with male grizzly bears having more 12-h (crepuscular) episodes than both female grizzly bears (t = 3.10, P = 0.002) and male black bears (t = 4.47, P < 0.001). However, we did not observe a difference in period between female grizzly bears and male black bears (t = 1.86, P = 0.065). For all bear categories, there was a difference between the occurrences of 12- and 24-h periods for some time intervals, with 12-h periods occurring more from mid-June to mid-August (F12,240 = 6.46, P < 0.001).
The percentage of bear-years that have 12-h versus 24-h periods by biweekly time interval for a) male black bears (Ursus americanus), b) female grizzly bears (Ursus arctos), and c) male grizzly bears, Yellowstone National Park, Wyoming (2007–2009). Sample size (n) for each time interval is given above each bar.
We observed a high degree of variability in onset and offset times of activity between individuals of both species and sexes (Fig. 3). However, all bears, regardless of time interval, became active before civil twilight irrespective of whether they were crepuscular or diurnal. Activity onset occurred before sunrise throughout the changing daylight hours and did not differ between grizzly bears and black bears (2.3 h ± 0.2 SD for grizzly bears and 2.6 ± 0.7 h for black bears, F1,31 = 1.06, P = 0.305). However, activity offset occurred before sunset for black bears (0.9 ± 1.2 h) but afterward for grizzly bears (0.6 ± 2.3 h, F1,31 = 1.06, P < 0.001). During the time intervals with the highest temperatures, 78% of grizzly bears and 36% of black bears rested during midday.
The hour of onset (open symbols) and offset (solid symbols) for individual a) male black bears (Ursus americanus), b) female grizzly bears (Ursus arctos), and c) male grizzly bears, Yellowstone National Park, Wyoming 2007)–2009), as they vary by time interval. During intervals when a bear is crepuscular 2 times are shown for onset and offset.
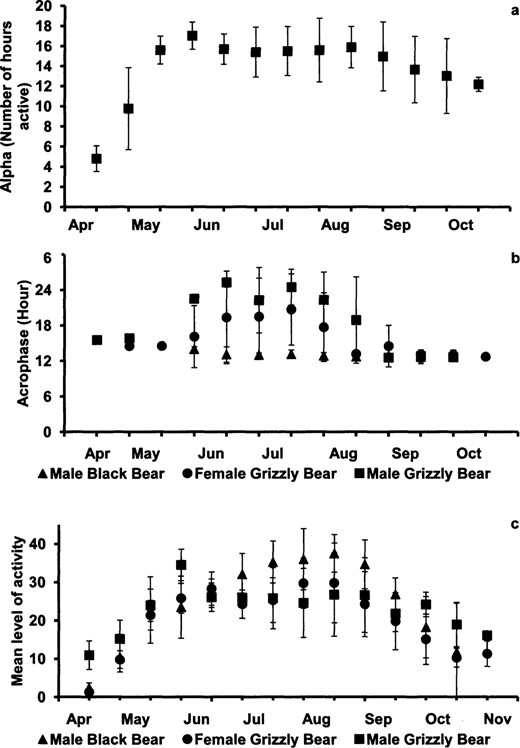
We did not observe a difference in duration of activity (alpha values) among male black bears, female grizzly bears, and male grizzly bears (F2,198 = 1.16, P = 0.203) or among years (F2,198 = 0.81, P = 0.448). However, alpha changed between some of the 2-week time intervals because bears were less active in the spring than at all other times (F12,198 = 5.02, P < 0.001; Fig. 4a). The peak of the activity rhythm (acrophase) differed between male black bears (1254 h ± 0034 SD) and both female (1558 ± 0502 h; t = −3.38, P = 0.001) and male grizzly bears (1826 ± 0545 h; t = -5.79, P < 0.001). The acrophases also differed between male and female grizzly bears (t = −3.06, P = 0.003). Acrophases for black bears did not change between spring and fall (F8,39 = 1.37, P = 0.249), but changed for both female (F11,61 = 2.28, P = 0.024) and male (F10,35 = 5.20, P < 0.001) grizzly bears during the summer when it shifted to later in the day (Fig. 4b).
Seasonal patterns in a) alpha for all bears (the length of time active during a 24-h period), b) acrophase (the peak hour of activity), and c) mean level of activity in Yellowstone National Park, Wyoming (2007–2009).
The mean level of activity was higher for male black bears (27.4 ±11.1 SD) than for both female (22.6 ± 9.4; t = 4.25, P < 0.001) and male (24.1 ± 8.0; t = 3.06, P = 0.002) grizzly bears. Male and female grizzly bears did not differ in their mean level of activity (t = −1.01, P = 3.313). Mean activity increased from spring through summer before decreasing in the late fall for all bears (male black bears: Fl3,73 = 10.56, P < 0.001; female grizzly bears: F113,117 = 7.21, P < 0.001; male grizzly bears: F13,85 = 1.99, P = 0.034; Fig. 4c).
Feeding time.—Feeding activity for all foods occurred more during daylight hours for black bears (berries: 74%; false-truffles: 84%; insects: 69%; pine nuts: 85%; graminoids: 69%; forbs: 72%; ungulates: 100%) but varied by food type for grizzly bears (male black bears: F1,12 = 4.34, P = 0.05; grizzly bears: F1,20 = 1–79, P = 0.196). Grizzly bears used berries (69%; F1,20 = 6.75, P = 0.009), false-truffles (76%; F1,20 = 20.06, P < 0.001), insects (72%; F1,20 = 15.21, P < 0.001), pine nuts (73%; F1,20 = 32.24, P < 0.001), and roots (76%; F1,20 = 49.09, P < 0.001) more during daylight hours than nighttime hours; however, graminoids (52% daylight; F1,20 = 0.25, P = 0.617), forbs (51% daylight; F1,20 = 0.26, P = 0.612), and ungulates (53% daylight; F1,20 = 0.21, P = 0.647) were used roughly equally during daylight and nighttime hours.
Discussion
Activity patterns varied between individuals of the same species, but the greatest differences in patterns were seen between grizzly bears and black bears, between males and females, and between seasons. We observed that grizzly bears gradually shifted their temporal niches in a nonrandom fashion during the active season, shifting from nocturnal to crepuscular to diurnal (Fig. 1). In contrast, sympatric black bears remained diurnal (Fig. 1). This temporal shifting in grizzly bears would probably not be as apparent when using traditional methods of analysis in which average activity values over several days, weeks, or months are used. Interestingly, grizzly bears are diurnal in captivity and alter their activity patterns during the lengthening days of spring and then extend these beyond the available daylight hours (despite shortening day lengths) during hyperphagia in the fall (Ware et al. 2012). This diurnal activity pattern can be shifted to a nocturnal one when food availability is restricted to the nighttime (Ware et al. 2012).
Based on the anticipatory rise in activity prior to sunrise, we hypothesize that entrainment of an endogenous rhythm by light allows bears to anticipate both daily and seasonal changes in sunrise and sunset times (Aronson et al. 1993; Schwartz et al. 2001). In addition, other nonphotic cues could be used to time activity depending on feeding times or food availability (Mistlberger 1994; Jansen et al. 2012). Thus, rather than being restricted to a fixed temporal niche, perhaps the coupling of 2 (or more) biological clocks in the grizzly bear allows a seasonal adjustment of activity patterns appropriate to the individual's needs. Pittendrigh and Daan (1976) proposed that seasonal adaptations are facilitated by a 2-oscillator system, although this has yet to be formally tested in bears. Nevertheless, several aspects of bear activity patterns would appear consistent with the 2-oscillator theory: the duration of activity increased for all bears upon emergence from hibernation when day lengths were increasing and typically less food was available; and the overall low level of activity in the early spring, presumably caused by the transition between the decreased metabolism of hibernation relative to that of the active season, then became entrained to a diurnal pattern once stable food supplies were available.
Although certain similarities between the behavior of captive and wild grizzly bears have been observed, some caution is required in assuming equivalence. Indeed, numerous instances of unpredictable patterns in wild groups of animals compared to captive (laboratory) individuals have been observed. For example, several rodent species that are primarily nocturnal in the laboratory exhibit diurnal or crepuscular patterns when placed in outdoor environments or when compared to their wild counterparts (Hoogenboom et al. 1984; Perrigo 1987; Daan et al. 2011). Others have been observed to switch their temporal niches (Eriksson 1973; Kronfeld-Schor and Day an 2003). Given the diversity of food types used by grizzly bears (Craighead et al. 1995; Fortin et al. 2013) and the diversity of terrain inhabited, it is reasonable to predict that food availability and food type play important roles in shaping behavior. Regardless of the precise mechanisms involved, the current results highlight the importance of interpreting laboratory (captive) findings with caution unless data from wild populations can be used for comparison.
Mean activity levels were higher for black bears than for grizzly bears. Grizzly bears also are generally more carnivorous and therefore consume a more energy- and nutrient-dense diet than do the more herbivorous black bears (Welch et al. 1997; Rode et al. 2001; Robbins et al. 2004; Fortin et al. 2013). For example, meat provided 23% ± 7% SD of the protein in the current black bear diets as compared to 45% ± 22% for male grizzly bears and 38% ± 20% for female grizzly bears in Yellowstone National Park (Fortin et al. 2013). In addition, black bears relied more on insects and small mammals, whereas grizzly bears fed more heavily on elk. The increased time necessary to meet energy requirements when foraging on smaller or lower-quality foods would presumably require increased activity levels; however, activity sensors were not calibrated to an individual bear's behavior and thus higher activity counts corresponding to increased activity levels needs to be confirmed.
Although the peak time of activity (acrophase) differed between grizzly bears and black bears during the summer, they overlapped in late spring and fall. Likely this is due to black bears not avoiding activity during daylight hours even when grizzly bears are active (Larivière et al. 1994; MacHutchon et al. 1998; Holm et al. 1999), which is consistent with other species that rarely switch activity phase because of competition (Kronfeld-Schor and Dayan 2003). Similarly, black bears may be active when grizzly bears are active because both species used dispersed, nonoverlapping, and nondefendable food resources (i.e., roots, berries, and pine nuts) in the late summer and early fall that might be best identified and exploited during daylight hours; and black bears might be able to sense and avoid grizzly bears spatially during daylight by using different habitats (Fortin 2011; Fortin et al. 2013).
Perhaps most surprising was the competency with which grizzly bears displayed a shift between nocturnal and diurnal activity patterns, which did not occur in any of the black bears. Although both female and male grizzly bears have exhibited nocturnality in response to anthropogenic influences (Gibeau et al. 2002; Nevin and Gilbert 2005; Kaczensky et al. 2006; Moe et al. 2007), we saw temporal shifting of individual bears that were far from human development. Interestingly, Scandinavian brown bears were crepuscular and nocturnal, perhaps to avoid encountering humans, even when feeding primarily on berries (Moe et al. 2007). Avoidance of humans is a factor in both selection of habitat and temporal use of that habitat, especially in bear populations in areas with higher human impact, such as Europe (Martin et al. 2010; Ordiz et al. 2012). However, in our study, grizzly bears were nocturnal primarily during the late spring and early summer when they were feeding heavily on elk calves. Seventy percent of the elk calves killed by grizzly bears were found at nighttime GPS fixes, indicating that the kills occurred at dusk or during the night (Fortin et al. 2013). This may suggest that bears primarily use scent to find elk calves, and elk calves are either more likely to remain bedded at night or are easier to catch at night if they try to escape (French and French 1990; Gunther and Renkin 1990). Grizzly bears then became primarily crepuscular and diurnal during late summer and early fall when shifting to roots, berries, and whitebark pine nuts (current study). Munro et al. (2006) also observed that bears feeding on roots and berries were primarily crepuscular and diurnal.
All studies to date have characterized black bears and grizzly bears as having 1 type of activity pattern (i.e., diurnal, crepuscular, or nocturnal) for the duration of the active season in the absence of human activity. As in our study, black bears in other areas also were crepuscular and diurnal, except becoming nocturnal when influenced by humans (Larivière et al. 1994; MacHutchon et al. 1998; Holm et al. 1999; Beckmann and Berger 2003; Matthews et al. 2006; Schwartz et al. 2010). Although black bears sympatric with grizzly bears in Grand Teton National Park and northwestern Wyoming were diurnal, male grizzly bears were either crepuscular or nocturnal (Holm et al. 1999; Schwartz et al. 2010). In our study, temporal overlap occurred seasonally between male black bears and male grizzly bears. This may have occurred because black bears used more forested habitats and male grizzly bears more nonforested habitats, although their diets overlapped for important food items, such as whitebark pine nuts (Fortin 2011; Fortin et al. 2013). Unfortunately, we were unsuccessful in our attempts to collar female black bears and thus cannot draw any conclusions as to their niche occupancy or ability to shift. However, in Grand Teton National Park both male and female black bears sympatric to grizzly bears were more diurnally active (Schwartz et al. 2010).
In summary, North American grizzly bears appear highly flexible in the timing of their activity patterns. They may be diurnal, crepuscular, or nocturnal in various ecosystems and seasons (Craighead et al. 1995; Holm et al. 1999; Munro et al. 2006; Schwartz et al. 2010). Although grizzly bears may respond to human activity by becoming nocturnal (Gibeau et al. 2002; Nevin and Gilbert 2005), examination of our data indicates that individual grizzly bears have the capacity to shift their temporal niche when not heavily influenced by human activity. Somewhat surprisingly, black bears in Yellowstone National Park did not exhibit the same flexibility, suggesting that a diurnal niche is the preferred time for their activity. For grizzly bear management purposes, recommendations for human activities should incorporate the bear's inherent daily and seasonal temporal flexibility. We recommend that future studies analyzing activity patterns take advantage of fine daily temporal scales to more accurately determine if grizzly bears or black bears exhibit temporal shifting without altering population productivity (Rode et al. 2006, 2007). We also recommend controlled studies to calibrate activity sensors to more fully understand differences between activity counts and behaviors, and to avoid the need for binning data into a binomial of active versus inactive.
Acknowledgments
Funding for this project was provided by United States Geological Survey Biological Resource Discipline Research (Natural Resources Preservation Program), United States Geological Survey Northern Rocky Mountain Science Center Interagency Grizzly Bear Study Team, Bear Center Washington State University, and Bear Management Office of the Yellowstone Center for Resources Yellowstone National Park. Personnel support was provided by Bear Management Office, the Interagency Grizzly Bear Study Team, and Washington State University. We thank C. Whitman, C. Dickensen, J. Ball, G. Rasmussen, and S. Thompson for their trapping effort and T. Coleman, S. Ard, M. Haroldson, K. West, S. Podruzny, G. Monroe, T. Rosen, J. Erlenbach, K. Quinton, C. Rumble, G. Wilson, R. Mowry, C. Wickhem, B. Fitzpatrick, S. McKenzie, P. Cross, N. Counsell, and K. Miller for help with sample collection. We also thank the many national park rangers who assisted us in many ways, from occasional transportation to monitoring our safety. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the United States Government.
Literature Cited
Author notes
Associate Editor was I. Suzanne Prange.