-
PDF
- Split View
-
Views
-
Cite
Cite
Tiago Y. Andrade, Wibke Thies, Patrícia K. Rogeri, Elisabeth K. V. Kalko, Marco A. R. Mello, Hierarchical fruit selection by Neotropical leaf-nosed bats (Chiroptera: Phyllostomidae), Journal of Mammalogy, Volume 94, Issue 5, 15 October 2013, Pages 1094–1101, https://doi.org/10.1644/12-MAMM-A-244.1
Close - Share Icon Share
Abstract
It is crucial to understand how Neotropical leaf-nosed bats select fruits, because their choices strongly affect the seed dispersal process, especially of pioneer plants. We tested the hypothesis of hierarchical fruit selection by phyllostomid bats at the levels of the bat genus and species by combining a literature database with field experiments. Considering our database for the whole Neotropics, Artibeus bats focus on Ficus (Moraceae) and Cecropia (Cecropiaceae), Carollia bats on Piper (Piperaceae), and Sturnira bats on Solanum (Solanaceae). Results from a field experiment in Brazil corroborated those preferences, because bats of those 3 genera selected 1st the fruits of their preferred plant genera, even when secondary fruits were offered in higher abundance. In another field experiment in Panama, we observed that 2 sympatric Carollia species, although focusing on the same plant genus, prefer different Piper species. Literature records for the whole range of the 2 Carollia species show that they have strongly nested diets. Our findings corroborate the hypothesis that frugivorous phyllostomids do not forage opportunistically, and, moreover, segregate their diets hierarchically at the genus and species levels.
Resumo
É crucial entender como morcegos filostomídeos neo trapicais selecionam frutos, porque as escolhas deles influenciam fortemente o processo de dispersão de sementes, especialmente de plantas pioneiras. Testamos a hipótese de seleção hierárquica de frutos por morcegos filostomídeos nos níveis do gênero e espécie de morcegos, através de uma combinação de um banco de dados e experimentos de campo. Considerando o nosso banco de dados para toda a região Neotropical, morcegos Artibeus focam em Ficus (Moraceae) e Cecropia (Cecropiaceae), morcegos Carollia em Piper (Piper-aceae) e morcegos Sturnira em Solanum (Solanaceae). Os resultados de um experimento de campo no Brasil corroboraram essas preferências, pois morcegos desses 3 gêneros selecionaram os frutos dos seus gêneros preferidos em primeiro lugar, até mesmo quando frutos secundarios foram oferecidos em maior abundância. Em outro experimento de campo no Panamá, observamos que 2 espécies simpátridas de Carollia, apesar de focarem no mesmo gênero de plantas, preferem espécies de Piper diferentes. Registros da literatura para toda a área de distribuição geográfica dessas duas espécies de Carollia mostram que elas têm dietas fortemente aninhadas. Nossas descobertas corroboram a hipótese de que filostomídeos frugívoros nao forrageiam de maneira oportunista e, além disso, segregam suas dietas hierarquicamente nos níveis do gênero e da espécie.
Seed dispersal is a vital plant-animal mutualism (Fleming and Kress 2011), especially in the Neotropics, where most plant species depend on frugivores for dispersal service (Terborgh et al. 2002). It is crucial to understand how different disperser groups select fruits, because their decisions have strong effects on the reproductive success of plants (Fleming and Sosa 1994) and, ultimately, on the structure of plant communities (Levine and Murrell 2003). Phyllostomid bats (Chiroptera: Phyllostomidae) are one key seed disperser group in the Neotropics (Lobova et al. 2009) and have a unique diet among frugivores (Fleming and Kress 2011). Bats and birds together generate nearly 80% of the seed rain in some sites (Galindo-Gonzáles et al. 2000), and they play complementary roles (Muscarella and Fleming 2007). Plenty of information is available on fruit selection by birds at different taxonomic levels (Sallabanks 1993; Westcott et al. 2005; Schupp et al. 2010), including evidence from field experiments and assessments of evolutionary pressures (e.g., Jordano 1995). Nevertheless, most information on fruit selection by frugivorous bats comes only from studies on their natural diets (e.g., Mello et al. 2008), and from relatively few experiments (Kalko and Condon 1998; Korine and Kalko 2005).
To understand dietary diversification in phyllostomid bats, especially in comparison to dietary diversification in frugivorous birds, it is important to consider that specialized frugivory evolved only in a single lineage of Neotropical bats (Rojas et al. 2011). Consequently, Neotropical frugivorous bats, although feeding on at least 50 plant families, focus their diets on 5 main genera (Lobova et al. 2009). Thus, phyllostomid bats have rather similar diets compared to other frugivores, such as birds and mammals (Fleming and Kress 2011). Despite the similarity in the diet of phyllostomid bats, there are some important differences among them. For instance, it has been frequently assumed that bats of different genera prefer fruits of different genera. However, the hypothesis of bat genus-plant genus associations derives mainly from studies on natural diets (e.g., Gorchov et al. 1995; Giannini 1999; Mello et al. 2004; Aguiar and Marinho-Filho 2007; Lobova et al. 2009) or uncontrolled experiments (Bonaccorso and Gush 1987). Nevertheless, because differences are observed repeatedly in different sites, they are interpreted as a mechanism that allows the coexistence of ecologically similar bat species (Marinho-Filho 1991; Giannini and Kalko 2004). Therefore, it remains disputed whether the observed dietary patterns result from preference or opportunism. Furthermore, assuming as true the hypothesis that congeneric bat species focus their diets on the same plant genus, there should be finer differences that allow their coexistence, because many of them occur in sympatry (e.g., Carollia perspicillata and C. castanea—Thies and Kalko 2004). Congeneric bat species probably prefer different plant species of the same genus, or consume the same species in different proportions, although the core plants in their diets are similar in some places (as observed by Bonaccorso et al. [2006] and Sánchez et al. [2012]).
In the present study, we aimed at understanding how species of phyllostomid bats differentiate their diets hierarchically at distinct phylogenetic levels, by combining a literature database with field experiments. We tested 4 main hypotheses: the 3 most important genera of frugivorous phyllostomids (in terms of abundance, range size, and specialization in frugivory— Lobova et al. 2009; Mello et al. 2011) consume fruits of different plant genera; those bat genus–plant genus associations result from preference, not opportunism; bats of the same genus, although preferring the same plant genus, focus their diets on different species; and within the same bat genus, the diet of specialists represents a subset of the diet of generalists (nested diets).
Materials and Methods
Study areas.—The present study was carried out in 3 areas. Two of them are located in southeastern Brazil: the private reserve of the Federal University of São Carlos (hereafter UFSCar; 21°58′8″S, 47°52′22″W; São Carlos municipality) and Ilha do Cardoso State Park (hereafter Cardoso; 25°4′22″S, 47°55′23″W; Cananéia municipality). The 3rd area was Barro Colorado Island, in the field station of the Smithsonian Tropical Research Institute, Panama (hereafter BCI; 9°09′N, 79°51′W; Balboa municipality). The reserve of UFSCar encompasses 500 ha and has heterogeneous vegetation: cerrado (Brazilian savannah), gallery forests, plantations of Eucalyptus and Pinus, and agricultural areas (Paese 1997). Cardoso is a coastal island that extends over 16,100 ha and is covered by sand dunes, restingas (coastal vegetation on sandy soils), and dense lowland rain forest (Atlantic Forest—Noffs and Batista-Noffs 1982). BCI has about 1,600 ha and is covered with tropical moist and semideciduous forests at several successional stages (Foster and Brokaw 1990).
Genus–genus associations.—First, we wanted to test at a biogeographic scale the hypothesis that phyllostomid bats of different genera prefer fruits of different genera, which was initially proposed by Fleming (1986). We compiled information on the diet of Neotropical leaf-nosed bats from the literature, and built a database with 365 papers and 4,160 records of bat-fruit interactions. Each report that a given bat species fed on a given fruit species was considered a record. Our database was started with information provided in the Bat-Plant Online Database (Geiselman et al. 2002; Lobova et al. 2009), and then complemented with additional South American literature. For each interaction published, we recorded the bat species and the plant species involved. We focused our analysis on the 3 most important Neotropical genera of seed-dispersing bats: Artibeus, Carollia, and Sturnira (Lobova et al. 2009). For each genus, we counted how many records there were from different papers, associating it with each plant genus. Differences among the 3 bat genera were tested with a contingency chi-square test. We also tested for the preferences of each genus separately with a chi-square test.
Fruit selection by bats of different genera.—To test whether the genus-genus associations observed in the literature were a result of preference or opportunism, we conducted behavioral experiments at UFSCar and Cardoso, Brazil, from January to December 2008. We mistnetted (7 × 2.5 m, 32-mm mesh; Ecotone, Gdynia, Poland) frugivorous bats and exposed them to 1-night feeding experiments. Following experiments, bats were released at the site of capture. In our protocol, we followed guidelines for the use of mammals in research given by the American Society of Mammalogists (Sikes et al. 2011), and received sampling permits from the Chico Mendes Institute for Biodiversity Conservation (SISBIO 19335-1, 11093-1, and 11093-2). A camping tent (Trilhas & Rumos Ltd., Teresópolis, Brazil) was used as a flight cage (3 m wide × 2.1 m long × 1.8 m high). Bats flew freely in the tent and took fruits offered on twigs side by side (50 cm away from each other). Test subjects were caught between 1800 and 2000 h of the same night in which the experiments were conducted. Each individual bat was exposed during 30 min to primary fruits (those preferred, according to our literature database: Cecropia–Artibeus. Piper-Carollia, and Solanum–Sturnira) and secondary fruits (other fruit genera that are important in the diet). In the 1st setup (control), fruits were given in the same quantity, and we observed which kind of fruit was picked up 1st. In the 2nd setup (treatment), fruits considered as nonpreferred were 3 times more abundant than preferred fruits, so we could see if the supposedly preferred fruits would still be picked up 1st. The activity of each individual was filmed with a Mini-DV video camcorder (model HC-26 with NightShot function; Sony Inc., New York, New York) using infrared illumination. We recorded which fruit the bat chose and ate 1st. We also documented the bat's behavior toward the fruits.
Experiments were finished and bats were released, at the latest, 6 h after the initial transfer to the flight cage. If a bat did not feed within the first 30 min, it was immediately released and excluded from subsequent analysis. Differences in the selection of primary and secondary fruits were tested with a chi-square test with Yates' correction (Zar 1996).
Fruit selection by bat species of the same genus.—To test whether bats of the same genus feed on different fruit species of the same genus, we conducted fruit-choice experiments with 2 sympatric Carollia species on BCI, Panama, in a custom-made flight cage (4.0 × 4.5 × 2.1 m), from March 1994 to August 1997. Piper species produce spikelike infructescences (Dyer and Palmer 2004), but here we refer to them simply as fruits. First, we analyzed fecal samples from C. perspicillata or C. castanea to identify the fruits that they naturally consume. The samples were collected during the Ph.D. project of WT. Based on the list of consumed Piper species, we prepared choice trials. Bats were caught between 1800 and 1930 h the same night in which the experiments were conducted, and were released on the following night. For each experiment, ripe fruits of 2 Piper species were cut into equal-sized pieces and presented in equal numbers in petri dishes on the floor, 1 dish for each Piper species. When 2 bats were kept in the same cage, we duplicated the setup. Ripe Piper fruits were collected in the afternoon before the experiment. Because fruiting periods of Piper species did not fully overlap, we could not test all of the possible combinations of Piper species. For example, P. aequale and P. culebranum fruited at different times. Consequently, our experiments closely reflect the natural situation of Piper availability in the field on BCI. We repeated trials with the same combination of Piper species with 1–6 individual bats. Because Carollia did not readily feed when a person was present in the flight cage, we left the bats alone and returned every hour to count the remaining pieces of fruits and to add fresh ones to maintain the same proportion of fruits as in the beginning of the trial. Every hour, we switched the position of the dishes to minimize spatial learning by the bats. Experiments lasted from 2 to 6 h (X̄ = 4.1 h) depending on the numbers of available ripe fruits and the bats' readiness to feed. For analysis, we pooled all individuals that were tested with the same combination of Piper species. We measured selectivity by comparing the quantity of Piper fruits eaten in each species pairing. To establish a preference order of Piper species in the experiments we determined for each Piper species separately the percentage of bats that chose each species exclusively, that rejected the species, that took it as 1st or 2nd choice, or that took it with equal preference with another Piper species in the trial. For analysis, we pooled the bats to which each Piper species was offered. To test for differences in the choice of fruits between the 2 Carollia species, we pooled together the number of fruits consumed per bat species, and performed chi-square tests with Yates' correction for each experimental set with a pair of Piper species (Zar 1996).
Nested diets.—Using our literature database and field data from BCI, we tested the hypothesis that the diets of more specialized bat species represent subsets of the diets of generalist bats (nested diets). We built adjacency matrices, in which bat species or genera were rows and plant species were columns (vertices), and frugivory records (edges) were recorded in the cells. First, we calculated the Schoener index of niche overlap for the 2 species. Then we used the nestedness index based on overlap and decreasing fill (NODF; Almeida-Neto et al. 2008) to measure the degree of nestedness in the matrix, and estimated its significance with a Monte Carlo procedure (1,000 randomizations, null model Ce).
Statistical analysis.—To test our predictions, we used different kinds of statistical tests, as explained in the previous sections. All analyses followed the standards proposed by Zar (2009) and Manly (2007). Results are presented as average ± SD.
Results
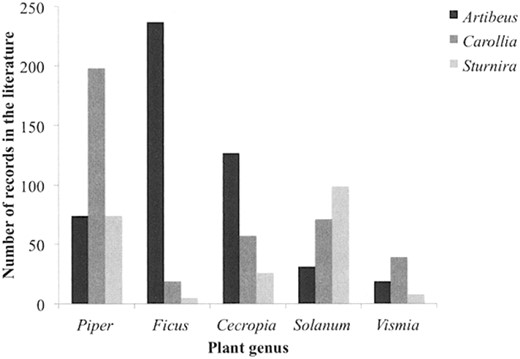
Genus-genus associations.—The analysis of our interaction database revealed that Artibeus, Carollia, and Sturnira bats were recorded feeding on 150 plant genera in the Neotropics. The 5 main plant genera consumed by the 3 bat genera were Piper (17%), Ficus (13%), Cecropia (10%), Solanum (10%), and Vismia (3%). Bats of the genus Artibeus had the broadest dietary range (116 plant genera), followed by Carollia (69 genera) and Sturnira (41 genera). When tested only for the 5 most consumed plant genera (Cecropia, Ficus, Piper, Solanum, and Vismia), most feeding records for Artibeus bats were composed of Ficus fruits (21% of all records, χ24=322.4, P < 0.001), those of Sturnira bats were composed of Solanum fruits (34%, χ24=166.3, P < 0.001), and those of Carollia bats were composed of Piper fruits (32%, χ24 = 258.9, P < 0.001; Fig. 1).
Records of frugivory compiled from the literature for the 3 main genera of Neotropical seed-dispersing bats (Artibeus. Carollia, and Sturnira) and the 5 main fruit genera consumed by phyllostomid bats (Cecropia. Ficus. Piper. Solanum, and Vismia).
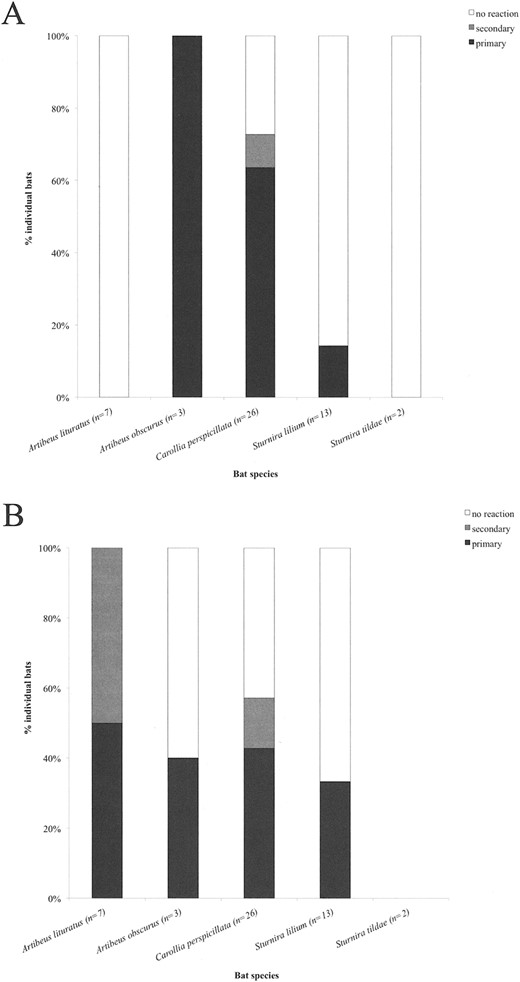
Fruit selection by bats of different genera.—We captured 270 bats of 16 species in UFSCar, and 63 bats of 13 species in Cardoso, Brazil. Sixty-nine bats from 5 frugivorous species were used in the experiment: Artibeus lituratus (n = 5), A. obscurus (n = 8), Carollia perspicillata (n = 34), Sturnira lilium (n = 18), and Sturnira tildae (n = 4). Of 69 bats, 26 responded to the experiment and were included in the analysis (Fig. 2). All of the bats flew on average 10 min around the fruits before they took them from flight or briefly landed on them and bit into them. C. perspicillata was the most responsive species, and selected Piper fruits 1st in both treatments. Most individuals took fruits off the branch and processed them on another tree branch. Overall, preferred fruits were consumed significantly more often than secondary fruits when both kinds of fruit were offered at equal abundance (91 % of the rounds; χ21 = 5.78, P = 0.02) and also when secondary fruits were 3 times more abundant (80% than preferred fruits; χ21 = 18.78, P < 0.001).
Results from fruit-choice experiments conducted in Ilha do Cardoso State Park and the reserve of the Federal University of São Carlos, Brazil. Number of times when each kind of fruit was selected 1st (chosen and then eaten) is given. A) Control with preferred and secondary fruits in equal abundances, and B) treatment with secondary fruits 3 times more abundant than preferred fruits.
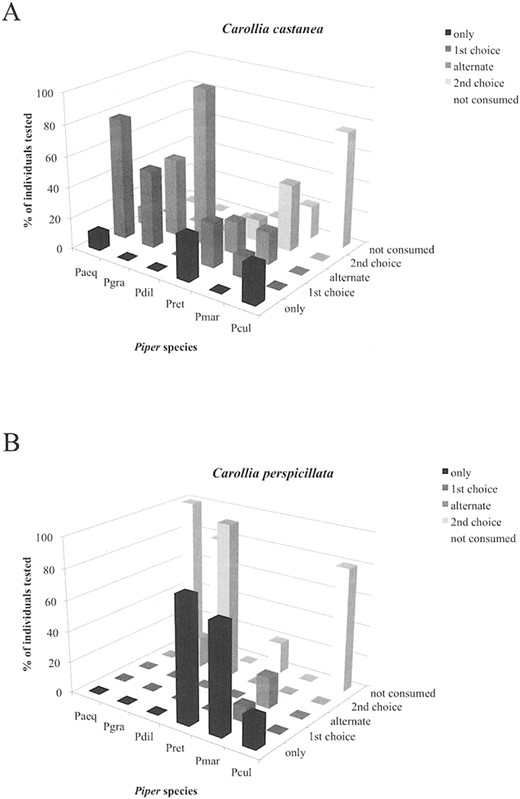
Fruit selection by bat species of the same genus.—On BCI, Panama, our results suggest a wider dietary range for C. perspicillata compared to C. castanea, with diets concentrated on different plant species (Table 1). The results of the fruit-choice experiment on BCI showed almost opposite preference orders for the 2 Carollia species (Table 2). For C. castanea, P. aequale was the most important species, because 11% of the tested individuals took this Piper species exclusively and two-thirds ate it 1st. In contrast to C. castanea, food selection in C. perspicillata was characterized by extremes (Fig. 3). All individuals rejected P. aequale, the preferred species of C. castanea. Most of the C. perspicillata took Piper reticulatum and P. marginatum exclusively, whereby P. reticulatum was preferred over P. marginatum.
Results from fruit-choice experiments conducted with Carollia bats on Barro Colorado Island, Panama. Proportion of individual bats of the species A) C. castanea (n = 22) and B) C. perspicillata (n = 16; y-axis) that selected each Piper species (x-axis) in a given preference order (z-axis). Abbreviations: Pcul = P. culebranum; Pmar = P. marginatum; Pret = P. reticulatum; Pdil = P. dilatatum; Pgra = P. grande; Paeq = P. aequale.
Natural diet of the 2 Carollia species studied on Barro Colorado Island, Panama. Numbers represent the total of fecal samples that contained each kind of food item. In total, 272 individuals provided us with 229 fecal samples (79 for C. perspicillata and 150 for C. castanea). Empty cells mean that no interaction record was obtained for the plant–bat pair.
| . | C. perspicillata . | C. castanea . |
|---|---|---|
| Genus Piper | ||
| P. aequale | 43 | |
| P. arboreum | 10 | 7 |
| P. carrilloanum | 2 | |
| P. cordulatum | 2 | 1 |
| P. culebranum | 1 | |
| P. grande | 1 | 22 |
| P. dilatatum | 14 | |
| P. hispidum | 1 | |
| P. marginatum | 5 | 3 |
| P. multiplinervia | 7 | 45 |
| P. reticulatum | 23 | 34 |
| Other plant genera | ||
| Vismia sp. 1 | 1 | |
| Vismia sp. 2 | 2 | |
| Dipteryx panamensis | 1 | |
| Spondias mombin | 2 | |
| Solarium sp. | 5 | 2 |
| Philodendron sp. | 3 | 3 |
| Anacardium excelsum | 9 | 2 |
| Ficus sp. | 1 | |
| Quararibea asterolepis | 3 | |
| Passiflora punctata | 1 | |
| Unknown 1 | 1 | |
| Unknown 2 | 5 | 1 |
| Unknown 3 | 1 | |
| Unknown 5 | 1 | 4 |
| Unknown 6 | 2 | |
| Other food | ||
| Pollen | 2 | |
| Insects | 1 | 1 |
| . | C. perspicillata . | C. castanea . |
|---|---|---|
| Genus Piper | ||
| P. aequale | 43 | |
| P. arboreum | 10 | 7 |
| P. carrilloanum | 2 | |
| P. cordulatum | 2 | 1 |
| P. culebranum | 1 | |
| P. grande | 1 | 22 |
| P. dilatatum | 14 | |
| P. hispidum | 1 | |
| P. marginatum | 5 | 3 |
| P. multiplinervia | 7 | 45 |
| P. reticulatum | 23 | 34 |
| Other plant genera | ||
| Vismia sp. 1 | 1 | |
| Vismia sp. 2 | 2 | |
| Dipteryx panamensis | 1 | |
| Spondias mombin | 2 | |
| Solarium sp. | 5 | 2 |
| Philodendron sp. | 3 | 3 |
| Anacardium excelsum | 9 | 2 |
| Ficus sp. | 1 | |
| Quararibea asterolepis | 3 | |
| Passiflora punctata | 1 | |
| Unknown 1 | 1 | |
| Unknown 2 | 5 | 1 |
| Unknown 3 | 1 | |
| Unknown 5 | 1 | 4 |
| Unknown 6 | 2 | |
| Other food | ||
| Pollen | 2 | |
| Insects | 1 | 1 |
Natural diet of the 2 Carollia species studied on Barro Colorado Island, Panama. Numbers represent the total of fecal samples that contained each kind of food item. In total, 272 individuals provided us with 229 fecal samples (79 for C. perspicillata and 150 for C. castanea). Empty cells mean that no interaction record was obtained for the plant–bat pair.
| . | C. perspicillata . | C. castanea . |
|---|---|---|
| Genus Piper | ||
| P. aequale | 43 | |
| P. arboreum | 10 | 7 |
| P. carrilloanum | 2 | |
| P. cordulatum | 2 | 1 |
| P. culebranum | 1 | |
| P. grande | 1 | 22 |
| P. dilatatum | 14 | |
| P. hispidum | 1 | |
| P. marginatum | 5 | 3 |
| P. multiplinervia | 7 | 45 |
| P. reticulatum | 23 | 34 |
| Other plant genera | ||
| Vismia sp. 1 | 1 | |
| Vismia sp. 2 | 2 | |
| Dipteryx panamensis | 1 | |
| Spondias mombin | 2 | |
| Solarium sp. | 5 | 2 |
| Philodendron sp. | 3 | 3 |
| Anacardium excelsum | 9 | 2 |
| Ficus sp. | 1 | |
| Quararibea asterolepis | 3 | |
| Passiflora punctata | 1 | |
| Unknown 1 | 1 | |
| Unknown 2 | 5 | 1 |
| Unknown 3 | 1 | |
| Unknown 5 | 1 | 4 |
| Unknown 6 | 2 | |
| Other food | ||
| Pollen | 2 | |
| Insects | 1 | 1 |
| . | C. perspicillata . | C. castanea . |
|---|---|---|
| Genus Piper | ||
| P. aequale | 43 | |
| P. arboreum | 10 | 7 |
| P. carrilloanum | 2 | |
| P. cordulatum | 2 | 1 |
| P. culebranum | 1 | |
| P. grande | 1 | 22 |
| P. dilatatum | 14 | |
| P. hispidum | 1 | |
| P. marginatum | 5 | 3 |
| P. multiplinervia | 7 | 45 |
| P. reticulatum | 23 | 34 |
| Other plant genera | ||
| Vismia sp. 1 | 1 | |
| Vismia sp. 2 | 2 | |
| Dipteryx panamensis | 1 | |
| Spondias mombin | 2 | |
| Solarium sp. | 5 | 2 |
| Philodendron sp. | 3 | 3 |
| Anacardium excelsum | 9 | 2 |
| Ficus sp. | 1 | |
| Quararibea asterolepis | 3 | |
| Passiflora punctata | 1 | |
| Unknown 1 | 1 | |
| Unknown 2 | 5 | 1 |
| Unknown 3 | 1 | |
| Unknown 5 | 1 | 4 |
| Unknown 6 | 2 | |
| Other food | ||
| Pollen | 2 | |
| Insects | 1 | 1 |
Selectivity of the bats Carollia perspicillata and C. castanea given as the percentage of fruits eaten of the 1st Piper species in the paired experimental rounds in the experiments carried out on Barro Colorado Island, Panama. Data from individual bats are pooled. Significant deviations from no selectivity (equal numbers of the 2 fruit types offered to the bats are taken) are indicated by an asterisk (*) based on results from chi-square tests (d.f. = 1, P < 0.05, with Yates' correction). Abbreviations: Pret = P. reticulatum; Pcul = P. culebranum; Paeq = P. aequale; Pdil = P. dilatatum; Pmar = P. marginatum; Pgra = P. grande; NA = not available.
| . | C. perspicillata . | . | C. castanea . | . | ||
|---|---|---|---|---|---|---|
| Fruit species . | Choice . | Selectivity . | No. bats . | Choice . | Selectivity . | No. bats . |
| Pret × Pcul | 66 × 16 | 0.80* | 3 | 17 × 18 | 0.49 | 3 |
| Pret × Paeq | 51 × 0 | 1.00* | 3 | 27 × 19 | 0.58 | 5 |
| Pret × Pdil | 16 × 3 | 0.84* | 1 | 26 × 3 | 0.90* | 2 |
| Pret × Pmar | 16 × 13 | 0.55 | 2 | 12 × 16 | 0.43 | 5 |
| Pmar × Paeq | 47 × 0 | 1.00* | 3 | 15 × 40 | 0.27* | 6 |
| Pmar × Pgra | 58 × 1 | 0.99* | 4 | 22 × 20 | 0.52 | 4 |
| Paeq × Pgra | NA | NA | NA | 8 × 3 | 0.73 | 2 |
| . | C. perspicillata . | . | C. castanea . | . | ||
|---|---|---|---|---|---|---|
| Fruit species . | Choice . | Selectivity . | No. bats . | Choice . | Selectivity . | No. bats . |
| Pret × Pcul | 66 × 16 | 0.80* | 3 | 17 × 18 | 0.49 | 3 |
| Pret × Paeq | 51 × 0 | 1.00* | 3 | 27 × 19 | 0.58 | 5 |
| Pret × Pdil | 16 × 3 | 0.84* | 1 | 26 × 3 | 0.90* | 2 |
| Pret × Pmar | 16 × 13 | 0.55 | 2 | 12 × 16 | 0.43 | 5 |
| Pmar × Paeq | 47 × 0 | 1.00* | 3 | 15 × 40 | 0.27* | 6 |
| Pmar × Pgra | 58 × 1 | 0.99* | 4 | 22 × 20 | 0.52 | 4 |
| Paeq × Pgra | NA | NA | NA | 8 × 3 | 0.73 | 2 |
Selectivity of the bats Carollia perspicillata and C. castanea given as the percentage of fruits eaten of the 1st Piper species in the paired experimental rounds in the experiments carried out on Barro Colorado Island, Panama. Data from individual bats are pooled. Significant deviations from no selectivity (equal numbers of the 2 fruit types offered to the bats are taken) are indicated by an asterisk (*) based on results from chi-square tests (d.f. = 1, P < 0.05, with Yates' correction). Abbreviations: Pret = P. reticulatum; Pcul = P. culebranum; Paeq = P. aequale; Pdil = P. dilatatum; Pmar = P. marginatum; Pgra = P. grande; NA = not available.
| . | C. perspicillata . | . | C. castanea . | . | ||
|---|---|---|---|---|---|---|
| Fruit species . | Choice . | Selectivity . | No. bats . | Choice . | Selectivity . | No. bats . |
| Pret × Pcul | 66 × 16 | 0.80* | 3 | 17 × 18 | 0.49 | 3 |
| Pret × Paeq | 51 × 0 | 1.00* | 3 | 27 × 19 | 0.58 | 5 |
| Pret × Pdil | 16 × 3 | 0.84* | 1 | 26 × 3 | 0.90* | 2 |
| Pret × Pmar | 16 × 13 | 0.55 | 2 | 12 × 16 | 0.43 | 5 |
| Pmar × Paeq | 47 × 0 | 1.00* | 3 | 15 × 40 | 0.27* | 6 |
| Pmar × Pgra | 58 × 1 | 0.99* | 4 | 22 × 20 | 0.52 | 4 |
| Paeq × Pgra | NA | NA | NA | 8 × 3 | 0.73 | 2 |
| . | C. perspicillata . | . | C. castanea . | . | ||
|---|---|---|---|---|---|---|
| Fruit species . | Choice . | Selectivity . | No. bats . | Choice . | Selectivity . | No. bats . |
| Pret × Pcul | 66 × 16 | 0.80* | 3 | 17 × 18 | 0.49 | 3 |
| Pret × Paeq | 51 × 0 | 1.00* | 3 | 27 × 19 | 0.58 | 5 |
| Pret × Pdil | 16 × 3 | 0.84* | 1 | 26 × 3 | 0.90* | 2 |
| Pret × Pmar | 16 × 13 | 0.55 | 2 | 12 × 16 | 0.43 | 5 |
| Pmar × Paeq | 47 × 0 | 1.00* | 3 | 15 × 40 | 0.27* | 6 |
| Pmar × Pgra | 58 × 1 | 0.99* | 4 | 22 × 20 | 0.52 | 4 |
| Paeq × Pgra | NA | NA | NA | 8 × 3 | 0.73 | 2 |
Nested diets.—On BCI, based on evidence from fecal analysis, C. perspicillata fed on 19 plant species, whereas C. castanea fed on 18 species. The calculated Schoener index of niche overlap between the 2 Carollia species was 0.39. Considering all literature records in the whole Neotropics for these 2 species, the difference in their diets was even larger, because C. perspicillata was recorded feeding on 155 and C. castanea on 38 plant species (their niche overlap index scored 0.21). The more specialized diet of C. castanea represented a subset of the more generalist diet of C. perspicillata, both in the local data from BCI (NODF = 0.61, P < 0.001) and in the literature data (NODF = 0.87, P < 0.001).
Discussion
In the present study we obtained evidence of hierarchical fruit selection by Neotropical leaf-nosed bats. The hypothesis that different genera of frugivorous bats prefer different plant genera was corroborated both with literature and experimental data. Furthermore, sympatric bat species of the genus Carollia, although preferring the same plant genus (Piper), differed in their selection of species of Piper. Our findings suggest that phylogeny plays an important role in determining bat diets and that more specialized phyllostomid bat species prefer fruits from within the pool of fruit species chosen by more generalist phyllostomid bats.
The information compiled from the data set on bat–fruit interactions confirmed preferences of different genera of Neotropical leaf-nosed bats for different plant families, as pointed out 1st by Fleming (1986) and corroborated by other studies (e.g., Marinho-Filho 1991; Giannini and Kalko 2004; Sánchez et al. 2012). Our experimental evidence supports the hypothesis that those selection patterns constitute real preferences, because preferred fruits were selected 1st, even when they were less abundant than secondary fruits, which corroborates the hypothesis that frugivorous bats forage in a nonopportunistic way (Korine and Kalko 2005), and suggests a rather tight relationship between bat genera and some plant groups (Lobova et al. 2009), which may ultimately be a result of diffuse coevolution (Heithaus 1982). It would be important to investigate the underlying physiological and sensory basis for the observed differences in dietary rankings. Chemical differences between plant families and genera may have led to different physiological adaptations in the genera of phyllostomid bats. For instance, the families Piperaceae and Solanaceae, which represent keystone resources in the diet of most frugivorous bats, differ strongly in the composition of secondary metabolites (Cipollini and Levey 1997; Dyer and Palmer 2004). Ultimately, the differences in dietary preferences result in a diversification of the seed dispersal services rendered by bats in the Neotropics, despite similarity and overlap observed, with particular plant groups relying on high-quality bat dispersal (see also Heer et al. 2010; Sánchez et al. 2012).
Considering that frugivorous phyllostomids are ecologically very similar and so play similar functional roles (Mello et al. 2011), small differences in fruit preferences may be interpreted as a mechanism that allow their coexistence, considering the available evidence on exploitative competition (Marinho-Filho 1991; Bonaccorso et al. 2006). Diversification of bat diets, although smaller if compared to diversification of bird diets, is probably important enough to have played a role in the diversification of Neotropical bats (Sánchez et al. 2010; Rojas et al. 2012).
The evidence obtained by analyzing the literature on the diet of 2 sympatric Carollia species, which was confirmed by our dietary study in the field and our behavioral experiments, indicates that more specialized bat species may have picked up their diets from the pool of more generalist species. In other words, relative specialists such as C. castanea and relative generalists such as C. perspicillata have nested diets. The nestedness in fruit selection could have resulted from the way frugivory evolved in phyllostomid bats. Because specialized frugivory evolved only once in a relatively small group of species (Rojas et al. 2011), it was likely easier for new species to start by feeding on a subset of the plant species already eaten by sister taxa. At the community level, nestedness may enhance the resilience of the whole interaction system (Bastolla et al. 2009), because the more sensitive specialists rely on resilient generalists, instead of being connected to other specialists.
In summary, our evidence suggests that phyllostomid bats, although depending on a narrow set of important plant genera, adopt strategies that ultimately diversify their seed dispersal services at different phylogenetic levels. Future studies should investigate the basis of the preferences observed. We suggest that, instead of thinking in terms of interaction syndromes (van der Pul 1972), we should investigate particular characters that seem to play a more important role. For instance, secondary metabolites (Cipollini and Levey 1997) and calcium content (Wendeln et al. 2000) seem to be keys to understand differences in fruit choice among Artibeus, Carollia, and Sturnira.
Acknowledgments
We thank many colleagues who helped us during the present study, in particular TYA's parents for their support. Our study was funded by the São Paulo Research Foundation (FAPESP 2006/00265-0, 2007/ 03405-0, and 2007/03415-6), the Alexander von Humboldt Foundation (AvH 1134644), and the Federal University of Minas Gerais (UFMG 2013/1). We dedicate our work to the memory of our mentor Elisabeth Kalko, who carried out the project together with us, but passed away before the manuscript was submitted.
Literature Cited
Author notes
Associate Editor was Matina C. Kalcounis-Rüppell.